
First Australian COVID-19 DNA vaccine trial commences
Led by the University of Sydney, Scientia Clinical Research (Sydney), Telethon Kids Institute (Perth) and the Women’s and Children’s Hospital (Adelaide), the COVALIA trial uses a gene-based vaccine with DNA sequences from the SARS-CoV2 virus.
The researchers have partnered with Australian biotech company Technovalia and its international vaccine partner BioNet who developed the DNA vaccine. It uses similar technology to other genetic vaccines, like mRNA, in use in Australia and internationally.
Gene-based vaccines use genetic (DNA) sequences from the virus. Researchers identify and isolate parts (genes) of the virus genome. Once the DNA is inside the cell the body uses the DNA code to make the coronavirus spike protein trigger an immune response.
This potential next-generation vaccine has no additives or preservatives. It will be given using a needle-free device that penetrates the skin with a jet spray and is designed to make sure the vaccine gets inside the cells to encourage good uptake by the immune system.
While not approved outside research studies in Australia, this needle-free device is already being used to give influenza vaccines in the United States.
About the Phase 1 trial
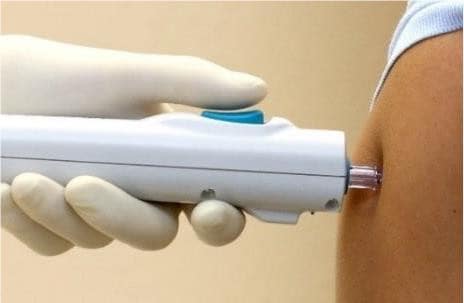
Needle-free device. Supplied by Pharmajet
The Phase 1 trial will recruit 150 participants with screening and enrolments of participants now open.
The key goal is to examine the safety of two doses of the vaccine given one month apart. If the trial is successful, then a larger phase 2 trial will be undertaken.
The trial will also look at whether a lower vaccine dose may work better following vaccination into the skin rather than muscle, which is important as it could enable more people to be vaccinated with the available vaccine supply.
Lead Investigator Associate Professor Nicholas Wood from the University of Sydney said: “We are very excited to start enrolment along with our colleagues in Perth and Adelaide, and to undertake the first needle-free COVID vaccine trial in Australia. The start of the COVALIA study is a significant milestone for all involved in this one-of-a-kind partnership between Australian institutions, the industry and the Australian Government with $3 million in funding from the Medical Research Future Fund (MRFF).”
Co-Investigator Professor Peter Richmond from the Telethon Kids Institute said, “It is really important that we continue to develop the next generation of COVID-19 vaccines, as we may see even better safety and immune responses. Having a greater range of vaccines available increases global vaccine capacity to ensure everyone has access to immunisation.”
Co-Investigator Professor Helen Marshall from the Women’s and Children’s Hospital and The University of Adelaide said “It’s a great opportunity for Australians to contribute to developing safe and effective COVID vaccines for the Australian population, and especially as it’s a needle free vaccination.”
More information
If you would like more information about participating in the trial please visit: http://www.scientiaclinicalresearch.com.au/covid-19-study/
Declaration: The trial has ethics approval. (Scientia: Bellberry Human Research Ethics Committee: 2021-01-008 [REGIS Application ID:2020ETH03350]) The researchers declare no competing interests. Collaborator Dr Anita Van Den Bigellaar from the Telethon Kids Institute is an advisor to Technovalia.