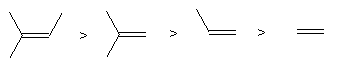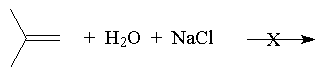
ALKENES
Reference: McMurry Ch 3 & 4, George et al Ch 1.2
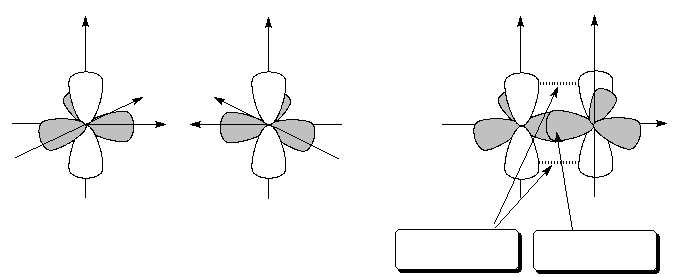
Structure and bonding
|
|
Bond length |
Bond strength |
|
C-C |
154 pm |
356 kJ mol-1 |
|
C=C |
133 pm |
636 kJ mol-1 |

|
|
Bond strength |
|
s bond |
356 kJ mol-1 |
|
p bond |
280 kJ mol-1 |
Nomenclature
Rules:
Examples:

IUPAC name of CH2=CH2 is ethylene, and of CH2=CHCH3 is propylene
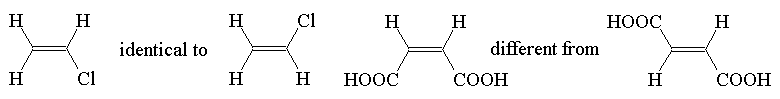
Constitutional isomers are possible, e.g. C4H8, (write names)

Stereoisomers are also possible as there is no rotation about the double bond. Stereoisomers of this sort are called diastereoisomers or diastereomers; they have different physical properties.
|
|
|
|
m.p. = -139 ° C |
m.p. = -106 ° C |
|
b.p. = 4 ° C |
b.p. = 1 ° C |
|
(Z)-2-Butene |
(E)-2-Butene |
Requirements for Alkene Stereoisomers
In general,
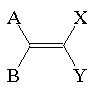
A ¹ B and X ¹ Y A may equal X or Y
B may equal Y or X
Example:
Example:
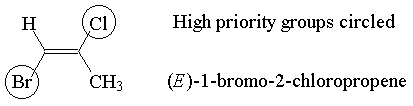
Preparation of alkenes
Reactions of Alkenes
General type of reaction is an electrophilic addition reaction. The electrons of the p bond are available for reaction, which has the net effect of replacing a p bond with a s bond and, therefore, is energetically favorable.
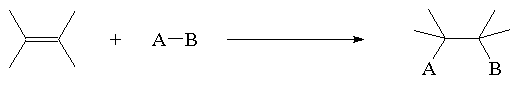
1. Hydrogenation – addition of hydrogen.
Example:
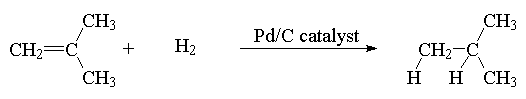
2. Halogenation
Example:
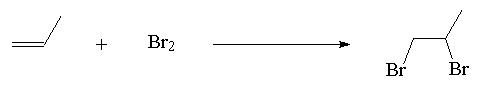
3. Hydrohalogenation
Example:
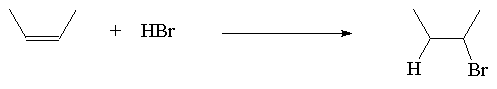
4. Hydration
Example:
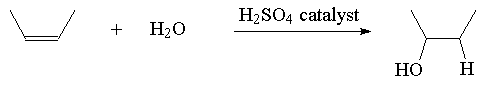
Classification of Reactions
It is convenient to classify reactions according to the overall type – addition, substitution, elimination and, where appropriate, the nature of the reagent which first interacts with the organic substrate. We need to define two new terms, which describe this nature: electrophile and nucleophile. These terms are complimentary, just like acid and base. Note the terms acid/base may also apply but generally have more limited use in organic chemistry.
Electrophile: A species that seeks and an electron pair
Nucleophile: A species that supplies and an electron pair
For example: H+ + H2O ® H3O+
H+ is the electrophile and H2O the nucleophile
Curly Arrow Notation
Curly arrow notation is used to indicate the movement of electrons in a chemical reaction and thus shows how bonds form and break. A "curly arrow" indicates the movement of an electron pair. It starts at the electron pair that moves and ends at the atom to which the electron pair has moved.
For example:

Here the lone pair on the oxygen is forming a new O-H covalent bond with the proton.
and similarly
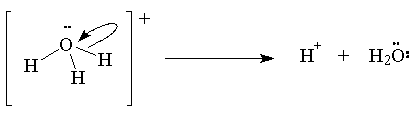
Here the O-H bond is breaking and the electron pair from the bond ends up as the lone pair on the oxygen atom of water.
Mechanism of the Electrophilic Addition Reaction
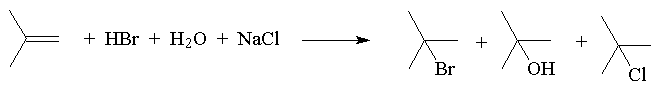
The addition of HX to a symmetric alkene gives only one possible product.
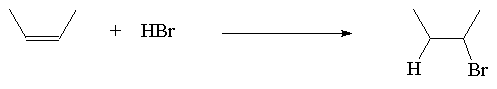
If an unsymmetrical alkene is used, there are two possible products, one of which dominates the product mixture.
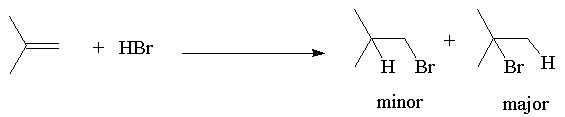
This was observed before it was understood and formulated as Markovnikov’s Rule: The hydrogen of an unsymmetrical reagent adds to the carbon of the double bond that has the greatest number of hydrogens already directly attached to it.
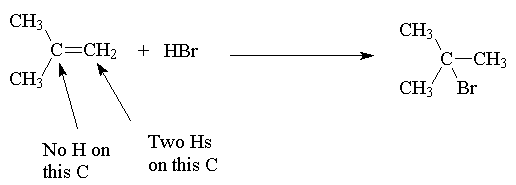
The explanation of this selectivity (regioselectivity) is as follows:
Reactants ® Intermediate ® Product
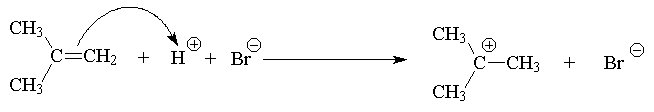
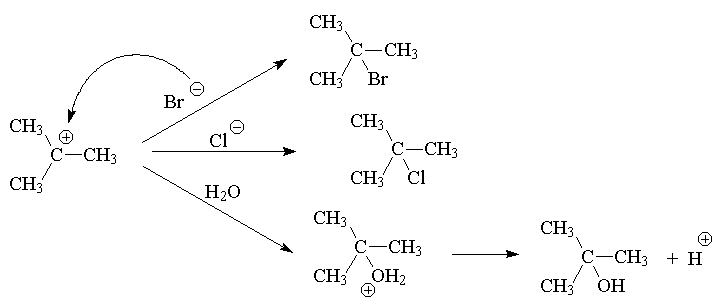
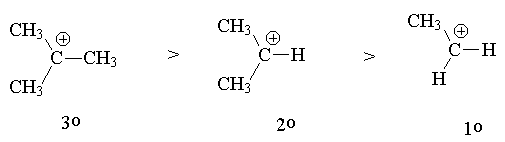
Hence: 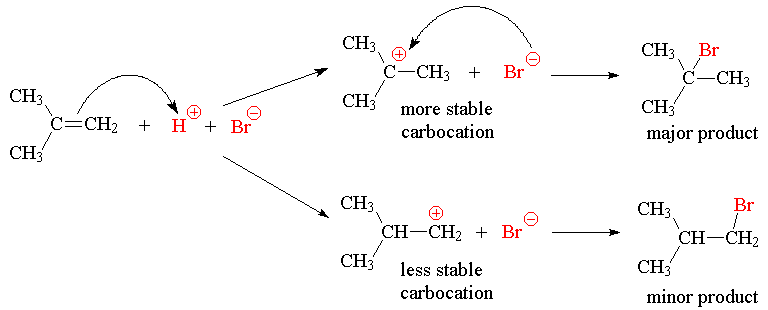
The evidence for the mechanism of the reaction is as follows: