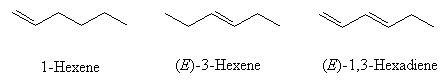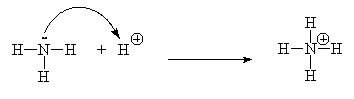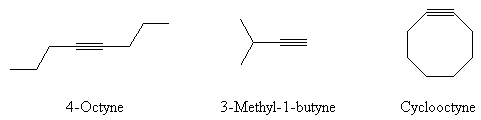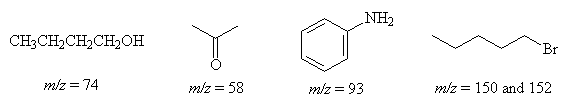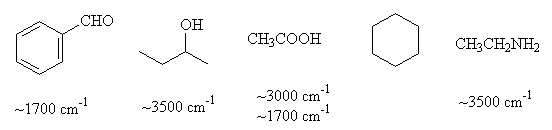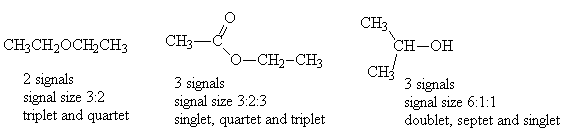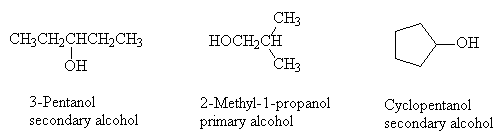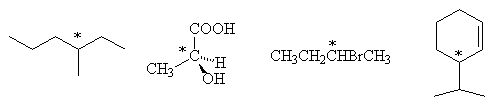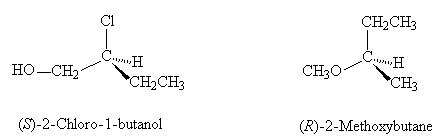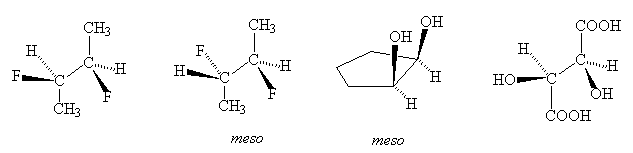ANSWERS
INTRODUCTION
- Name the functional groups in the following molecules:

Return to Questions
ALKANES
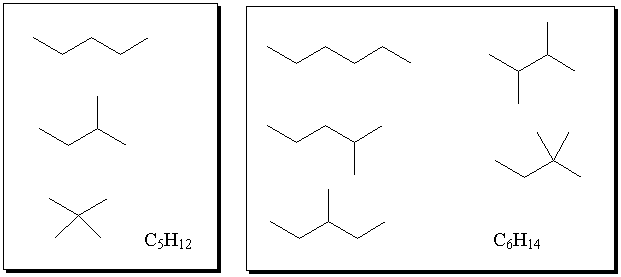
- Draw all the constitutional isomers of C5H12 and C6H14.
- Draw the Newman projections of the conformers representing the maximum and minimum points on the energy profile. Draw the energy profile of a butane molecule as the C2-C3 bond is rotated through 360° .

- Name the following compounds:
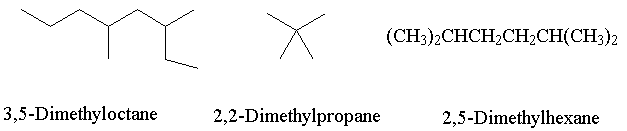
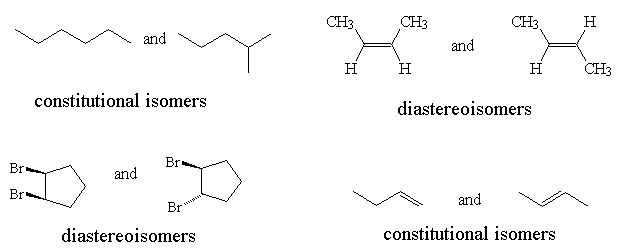
- Classify the following pairs of molecules as Constitutional isomers, Conformational isomers or Diastereoisomers.
Return to Questions
ALKENES
Give the names of the following molecules:
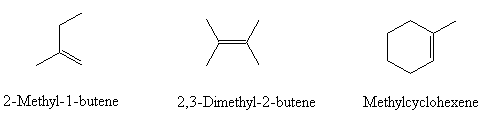
- Name these molecules (include Z and E where appropriate)

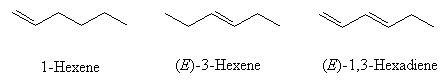
- Draw the curly arrow in the following reaction:
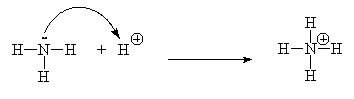
- Use arrow notation to illustrate the mechanism of the reaction of Br2 with propene. Label each species electrophile or nucleophile.
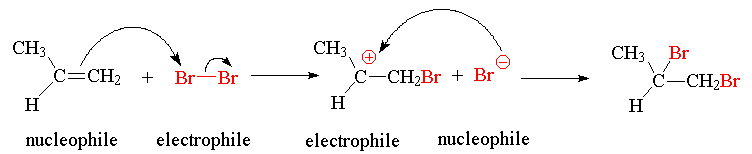
Return to Questions
ALKYNES
- Name the following compounds
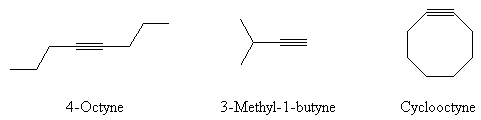
- Predict the products in the following reactions:
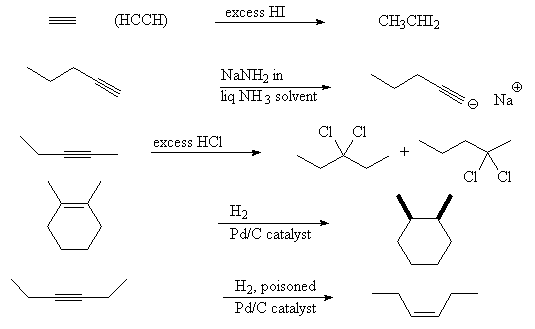
Return to Questions
AROMATIC COMPOUNDS
- Fill in the missing reagents or products in the following reactions:

Return to Questions
SPECTROSCOPY
What is the value of m/z of the molecular ions (integer values) of the following compounds?
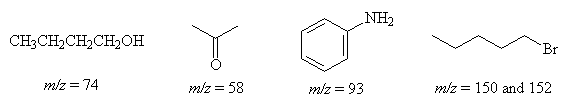
- Which of the following compounds strongly absorb electronic radiation in the ultraviolet-visible region of the spectrum ?

- Which of the compounds: CH3CH2COCH3, CH3CH2OCH3, CH3CH2CH2OH, is the following infrared spectrum representative of?
CH3CH2CH2OH - note O-H absorption around 3350 cm-1
- Indicate which of the following compounds absorb around 1700 cm-1 and around 3500 cm-1 in the infrared region.
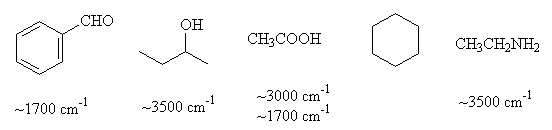
- A compound with molecular formula C3H8O has a strong absorption at 3600 cm-1 in the infrared spectrum. Give a reasonable condensed structural formula for any compound whose structure is consistent with this data.
CH3CH2CH2OH or CH3CH(OH)CH3
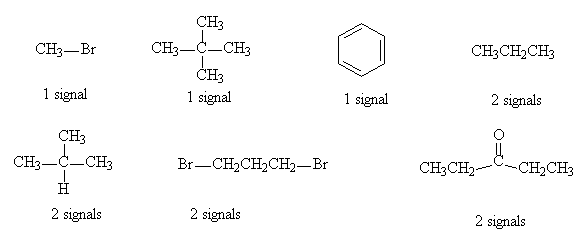
- How many signals would be expected in the 1H NMR spectrum of each of the following compounds?
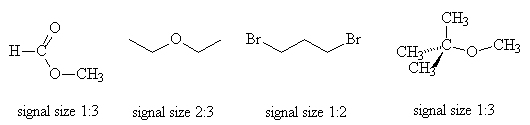
- Give the ratio of signal sizes observed in the 1H NMR spectrum of each of the following molecules:
- Give the number of signals, their relative intensities and the multiplicities in the 1H NMR spectra of each of the following compounds:
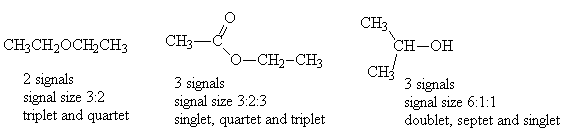
Return to Questions
ALCOHOLS
Name and classify the following alcohols as primary, secondary or tertiary
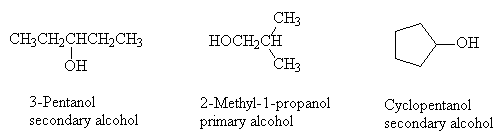
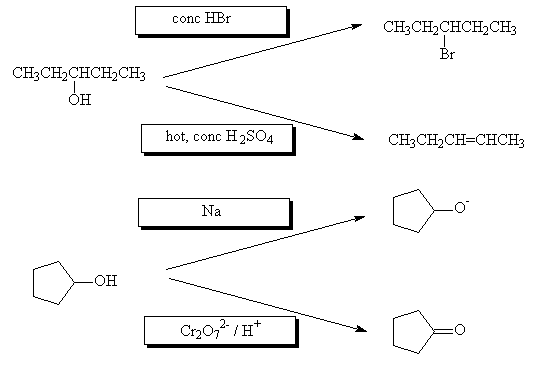
- What reagents are used in the following reactions?
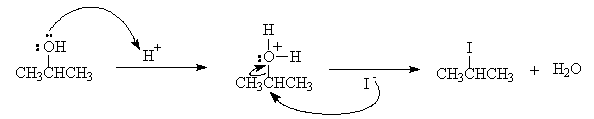
- Draw curly arrows to describe the nucleophilic substitution reaction of 2-propanol with concentrated HI to form 2-iodopropane
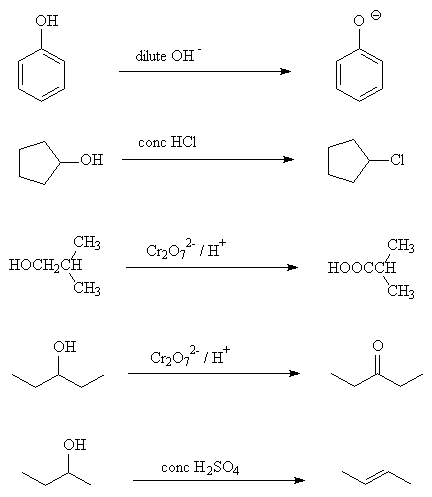
- Draw the product formed in the following reactions:
Return to Questions
ORGANIC HALOGEN COMPOUNDS
Predict the products from the following reactions:

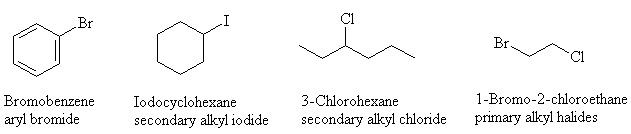
- Name and classify (1° , 2° , 3° or aryl) the following halogen compounds:
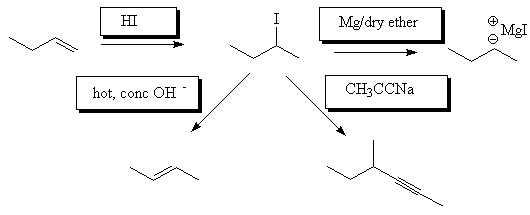
- Fill in the reagents in the following reaction scheme:
Return to Questions
ALDEHYDES AND KETONES
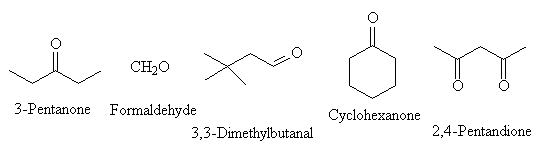
Name the following compounds
- Fill in the reagents in the following transformations:

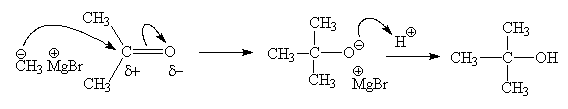
- Use curly arrow notation to indicate the mechanism of nucleophilic addition of methyl magnesium bromide to acetone followed by treatment with aqueous acid.
- Draw the products of the following reactions:

Return to Questions
CARBOXYLIC ACIDS
Name the following carboxylic acids
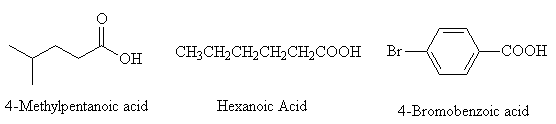
Return to Questions
CARBOXYLIC ACID DERIVATIVES
- Draw all the product(s) of the following reactions
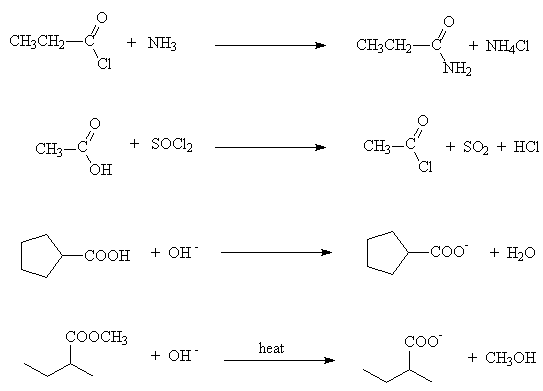
- Fill in the missing reagents
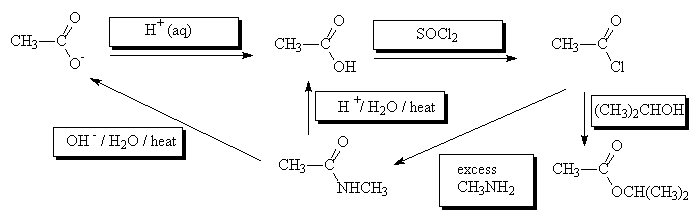
Return to Questions
ISOMERISM AND STEREOCHEMISTRY
- Classify the following isomers as constitutional, conformational or diastereisomers:
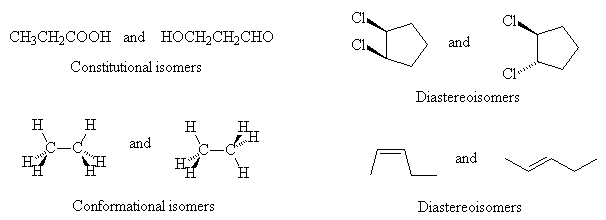
- Identify with an asterisk (*) the stereogenic carbon centres in the following molecules:
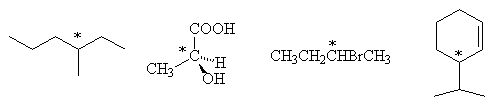
- Assign the configurations of the following stereogenic carbon centres:
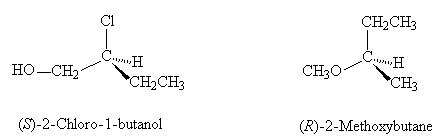
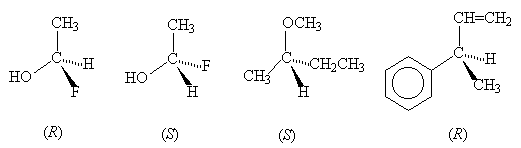
- Identify the configuration (R or S) of the stereogenic carbon centre in each of the following compounds:
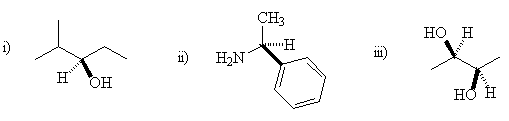
- Which of the following compounds is/are meso-compounds ?
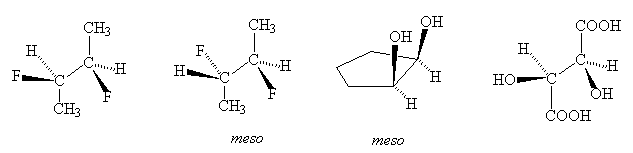
Return to Questions