AROMATIC COMPOUNDS
Reference: McMurry Ch 5 George et al Ch 1.3
Prime example of an aromatic compound is benzene, C6H6.
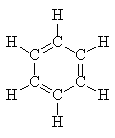
In 1865 Kekule proposed this structure:

However the chemical characteristics of benzene were unlike those of other alkenes, for example
|
|
|
|
|
H2 with Pd/C catalyst |
rapid reaction at room temp and pressure |
slow hydrogenation even at high temp and pressure |
|
Br2 |
rapid bromination |
no reaction |
Similarly, differences in the physical characteristics were observed. For example, in benzene, all carbon – carbon bond lengths and strengths are identical and intermediate between those of a double and single bond. Benzene is also about 140 kJ mol-1 more stable than predicted for 1,3,5-cyclohexatriene.
|
|
Bond length |
Bond strength |
|
C-C |
154 pm |
356 kJ mol-1 |
|
C=C |
133 pm |
636 kJ mol-1 |
|
benzene |
139 pm |
518 kJ mol-1 |
Structure and bonding
There are two models of bonding in benzene
|
1. In the valence bond model each of the carbon atoms in benzene is sp2 hybridised and forms s-bonds to two neighbouring carbon atoms and a s-bond to one hydrogen. Each carbon atom has a p-orbital which can participate in p-bonding.
|
|
|
If the bonds were normal C=C bonds, the bonding in benzene could be drawn in either of two identical ways. The valence bond theory says that the bonding in benzene is best described as an average or "resonance hybrid" of the bonding arrangements which can be drawn. |
|
|
2. The bonding in benzene can also be described in terms of molecular orbital theory. Here the p-orbitals of the six sp2-hybridised carbon atoms overlap with each other to form a single continuous p-bond. The six electrons in this bond effectively form a toroidal electron cloud which lies above and below the plane of the carbon atoms. |
|
Irrespective of which theory is used to describe the bonding, benzene is a perfect hexagon where all the bond angles are 120° and where all six C-C bonds are identical.
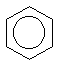
When drawing a benzene molecule, it is common to draw a circle in the centre of the hexagon of carbon atoms. This implies 'delocalisation' of the 6 p-electrons around the aromatic ring and indicates the real symmetry of the benzene molecule.
Nomenclature
There is not much that is systematic about the nomenclature of aromatic compounds. Unfortunately, the names have to be learnt!
Reactions of Benzene
The general type of reaction is an electrophilic substitution reaction. Like alkenes, an electron pair from a p bond is available for reaction. However, an addition reaction would result in loss of the "resonance energy" which makes aromatic compounds more stable than the analogous alkenes. Consequently substitution occurs and the net effect preserves the aromatic nature of the compound.
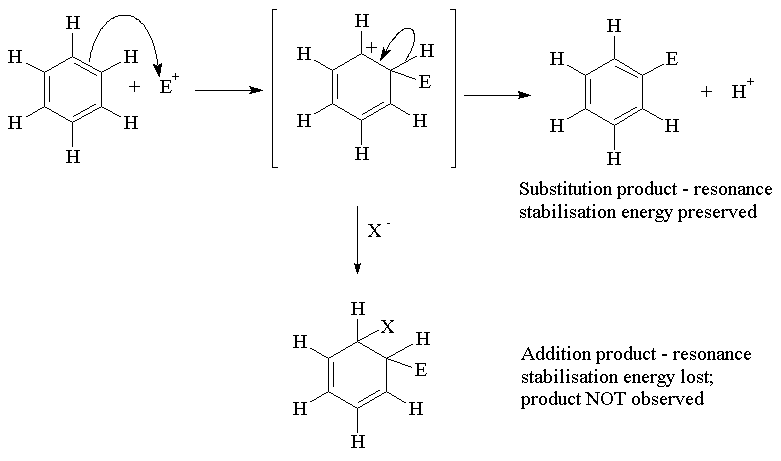
Aromatic hydrocarbons undergo substitution reactions only with extremely powerful electrophiles, which include the nitronium ion (NO2+), the bromonium ion (Br+) & the chloronium ion (Cl+) and acylium ion (RCO+). These electrophiles are too reactive to be stable species and are usually generated in situ (within the reaction mixture) immediately before they are required for aromatic substitution.
NO2+ is generated in a mixture of concentrated sulfuric and nitric acid ; Cl+ is generated by reaction of Cl2 with a Lewis acid such as AlCl3; and RCO+ is generated by reaction of RCOCl with AlCl3.

1. Nitration – substitution of H for NO2
Example:
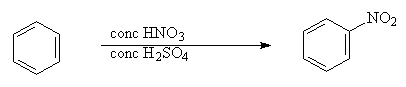
2. Halogentaion
Example:
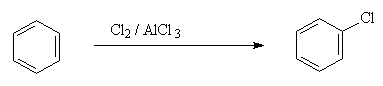
3. Friedel-Crafts acylation
Example:
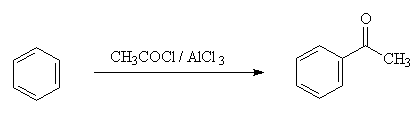
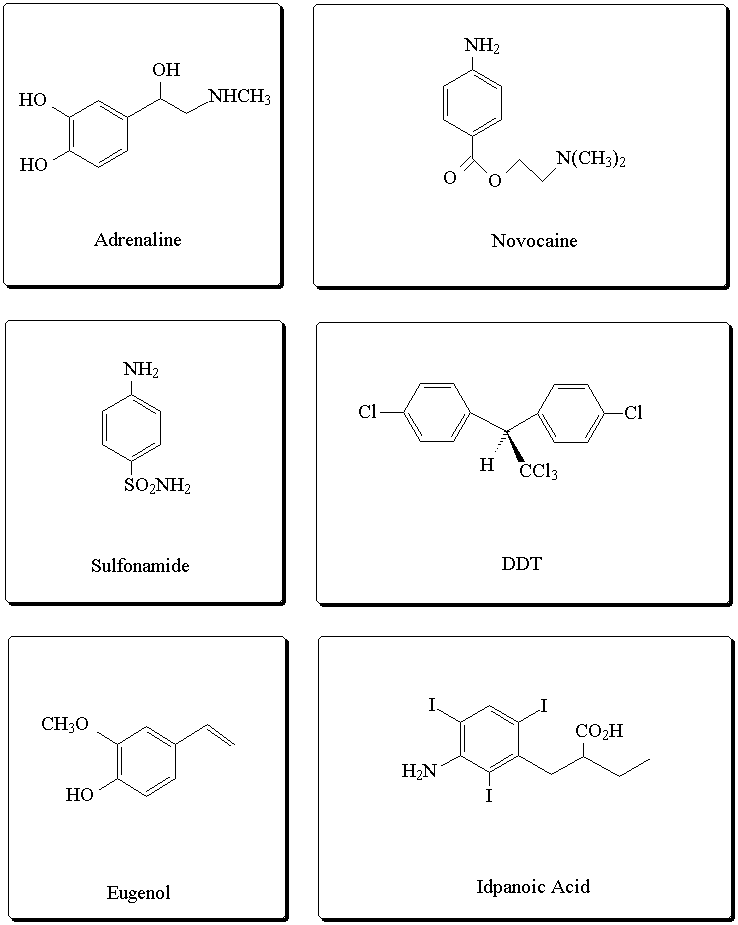
Some biologically active compounds containing an aromatic ring: