Extracellular vesicles
We focus our experiments on brain endothelial cells, which are central in immune-inflammatory disorders, and on clinical samples. We have shown that one of the important aspects of immunopathology is the release of extracellular vesicles (EV). These vesicles include microvesicles (previously called microparticles) and exosomes, which are nanosize elements, specifically between 10 and 800 nanometres.
We now investigate the precise role of these nanosize elements in immunoregulation and immunopathology, as well as their potential as biomarkers in a number of diseases. Our laboratory, called the Vascular Immunology Unit, performs the in vitro experiments on co-culture models based on brain endothelium, including the characterisation of extracellular vesicles (EV), which include microvesicles/microparticles and exosomes. Professor Grau brings his experience in Pathophysiology and Immunology since 1983 and, since 1999, in EV biology, as assessed by his 87 papers in the field of microparticles. We are also currently developing the evaluation of microvesicles as biomarkers of severity in various illnesses, notably cancer and multiple sclerosis. Together with Professor Peter Lay and his team, we have recently shown that microvesicles can be analysed by vibrational spectroscopy techniques (Lee et al, FASEB J, 2017 Jul;31(7):2817-2827).
Our project is part of a Sydney Nano Flagship Program (“New Insights into disease and drug targets: from endogenous biological nanoscale vesicles to engineered nanoparticles”) led by Professors Lay, Grau and King on nanomedicine, which is based on breakthrough discoveries in the bioinorganic chemistry and roles of EVs, particularly microvesicles (MVs) and exosomes, in disease pathologies. We’re working also with Professor Sorrell and Associate Professor Djordjevic (infectious diseases), Professor King and Emeritus Professor Hunt (pathology).
Professor Grau, in collaborations with Professors Sorrell, King and Hunt, has made innovative discoveries into key roles of MVs and the innate immune system in diseases. The innovation in the current research is to combine this expertise with new bionanospectroscopic protocols (X-ray, vibrational and fluorescence microscopies, and flow cytometry) to characterise these specific MVs within these heterogeneous mixtures and study their formation and specific biochemical stimuli/pathologies in target cells involved in disease processes. Ultimately this may lead to many applications in new and specific diagnostics and medical interventions.
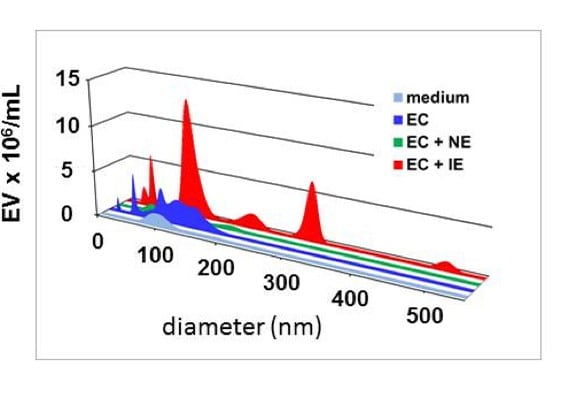
Imagen Nanosize tracking analysis of extracellular vesicles (EV)
Pictured below are the EV released by human brain endothelial cells (EC), upon co-culture with Plasmodium falciparum infected erythrocytes (IE). Non-infected erythrocytes (NE) are used as controls.
Recent publications
- El-Assad F, Wheway J, Hunt NH, Combes V, Grau GE. In vivo production, fate & pathogenicity of plasma microparticles in experimental cerebral malaria. PLoS Pathog 2014, 10(3):e1003839. doi: 10.1371/journal.ppat.1003839.
- Li Ccy, Eaton S, Young PE, Lee M, Shuttleworth R, Humphreys DT, Grau GE, Combes V, Bebawy M, Brammah S, Buckland ME, Suter CM. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in brain endothelial cells. RNA Biol. 2013 10: 1333-44. doi: 10.4161/rna.25281. Epub 2013 Jun 17.
- Wheway J, Latham S, Combes V, Grau GER. Endothelial microparticles interact with and support the proliferation of T cells. J Immunol 2014 Sep 3. pii: 1303431.
- Latham SL, Tiberti N, Gookoolparsadh N, Holdaway K, Olivier Couraud PO, Grau GE, Combes V. Immuno-analysis of microparticles: probing at the limits of detection. Sci Rep. 2015 Nov 10;5:16314. doi: 10.1038/srep16314.
- Combes V, Latham SL, Wen B, Allison AC, Grau GER. Diannexin down-modulates TNF-induced endothelial microparticles by blocking membrane budding process. Int J Innov Med Health Sci 7: 1-11, 2016.
- Zinger A, Latham SL, Combes V, Byrne S, Barnett MH, Hawke S, Grau GE. Plasma levels of endothelial and B cell-derived microparticles are restored by fingolimod treatment in multiple sclerosis patients. Mult Scler J 2016 Mar 1. pii: 1352458516636959.
- Tiberti N, Latham SL, Bush S, Cohen S, Juillard A, Grau GER, Combes V. Exploring experimental cerebral malaria pathogenesis through the characterisation of host-derived plasma microparticle protein content. Sci Rep 2016 6:37871 | DOI: 10.1038/srep37871.
- Lee J, Wen B, Carter EA, Combes V, Grau GER, Lay PA. Infrared Spectroscopic Characterization of Monocytic Microvesicles (Microparticles) Released upon Lipopolysaccharide Stimulation. FASEB J 2017. Jul;31(7):2817-2827.
