University nanotechnology approved for “First in Human” trials
The Key Centre for Polymers and Colloids (KCPC) at the University of Sydney has recently licensed magnetic nanoparticle stabilisation technology to the University of South Australia spin off company, Ferronova P/L.
Ferronova has now received approval to use this technology in “first in human” trials at the Royal Adelaide Hospital, for sentinel lymph node detection in oral cancer patients.
These magnetic nanoparticles have already demonstrated better performance for this application compared to the current commercialised product in preclinical mouse and pig model studies over the past 3 years. If these trials are successful, they could offer vastly improved health outcomes for a wide range of cancer sufferers.
If these trials are successful, they could offer vastly improved health outcomes for a wide range of cancer sufferers.
Sentinel lymph node biopsy is a procedure used in some types of cancer, including head and neck, prostate, breast, colon, cervix, and lung, to determine whether cancer cells have spread from a primary tumour to nearby lymph nodes or possibly other organs.
The standard procedure requires the use of a radioactive substance to identify the lymph nodes, which can then be removed by a surgeon. However, this process often produces false negatives, exposes the patient and medical staff to radiation and must be carried out in a nuclear medicine facility.
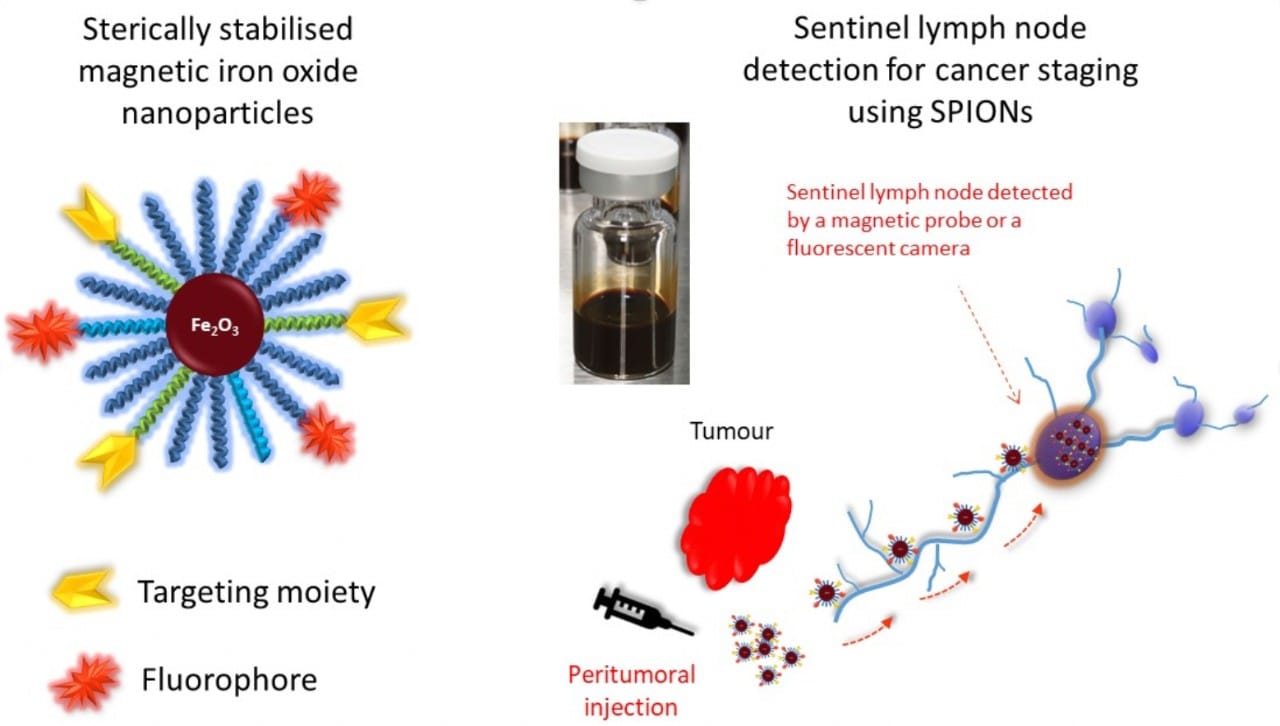
The KCPC magnetic iron oxide nanoparticles will be used in this procedure to increase the accuracy of lymph node detection without radiation exposure. The nanoparticles are injected around primary tumours where they then travel to nearby lymph nodes. The lymph nodes are then detected using intraoperative surgical magnetic detectors or infrared cameras.
The promise of nanomedicine
Twenty years ago, nanomedicine was thought to hold great promise and generated much excitement regarding its potential to revolutionise the treatment of cancer and provide enhanced imaging capabilities.
However, problems such as poor stability and aggregation within the body, leading to dose limiting fibrosis have appreciably reduced the enthusiasm of researchers for nanoparticles in medicine. The steric stabilising technology developed within the KCPC prevents the nanoparticles from aggregating.
KCPC research in animal studies has shown that their sterically stabilised nanoparticles remain stable and are eliminated from the body without adverse side effects and toxicity following procedures. The number of steric stabilising polymer chains on each particle is easily controlled, as is the proportion of these polymer chains that have a functionalisable end group. These functionalisable groups are used to attach targeting agents, fluorophores and other desired moieties. See schematic below:
20 years of advancement
The Brian Hawkett group within KCPC has been working with sterically stabilised superparamagnetic iron oxide nanoparticles for the past 20 years.
Their steric stabilisers are short chain diblock copolymers, with one block that is designed to grab onto the surface of the particle and the other block is very soluble in the dispersion medium. Thus, they are able to stabilise almost any sort of particle in almost any sort of medium, by changing the nature of one or both polymers.
This technology has proven to be adaptable across a diverse range of applications including:
- Explosive emulsions, funded by Dyno Nobel Asia Pacific and the ARC, in collaboration with Professor Greg Warr, also of KCPC. The particles were incorporated into very monodisperse saturated aqueous ammonium nitrate droplets dispersed in oil, to enable chaining of those droplets in a magnetic field.
- Ionic liquid ferrofluids for a project in collaboration with Professors Brad King of Michigan Tech and Juan de la Mora of Yale, and funded by AOARD and the US Air Force. These fluids were used in a space propulsion application for mini satellites.
- The biomedical application of this technology was developed with Sirtex Medical over a period of 17 years. However, this work was discontinued due to a company restructure and the subsequent sale of Sirtex to a Chinese consortium in 2017.
- Zeta Therapeutics P/L have also licenced the use of this technology with the aim to improve treatment outcomes for women suffering from ovarian cancer.