
Disentangling the evolved brain from its primitive state
A threat to your life triggers a primal survival response from your body – your heart races, muscles tense up and breathing fastens. A cascade of hormonal signals also trigger your body to sweat, be alert and boost energy levels.
At any moment, our bodies are ready to trigger these instinctual behavioural responses. Though we’re unlikely to face such threats in our daily lives, these survival mechanisms are instilled into our nervous system. At the heart of this lies Maclean’s triune-brain theory, stating that the neocortex (our modern brain) evolved from an ancestral ‘reptilian’ core.
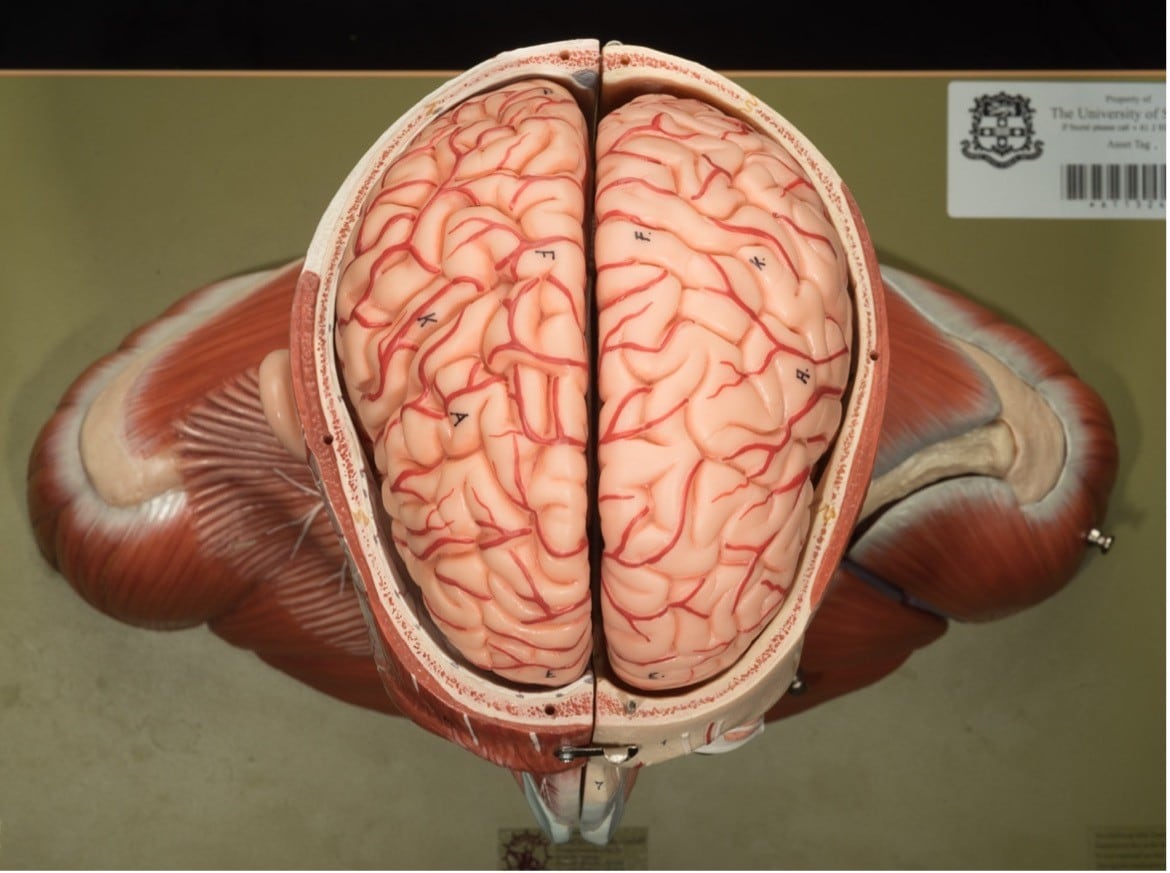
Superior view of an anatomical model revealing the cerebral cortex
Primitive neural structures
For structures regulating basic survival functions, their anatomy is highly conserved across vertebrate species. One such structure is the midbrain periaqueductal gray (PAG) sitting at the dorsum (Latin for “back”) of the brainstem.
In the 1990’s, Professor Kevin Keay from the University of Sydney pioneered research into the PAG and its role in fight-or-flight, pain and defence mechanisms.
Recently, a structure corresponding to the mammalian PAG has been revealed in the sea lamprey (Petromyzon marinus). The lamprey constitutes the most primitive of all vertebrate species, with records being dated back 300 million years ago.
Its anatomical location and connections show a high degree of homology with the mammalian PAG as well as its zebrafish counterpart, the griseum centrale. It’s curious that this survival circuit, having sustained life, is retained in the phylogenetically oldest group of vertebrates.
Understanding what makes our brains unique
Unlike any other animal, humans have developed higher order cognition capable of reasoning, problem-solving and selective attention. In part, over time, our brain size has expanded into this ‘neocortex’ which drives such cognitive faculties.
Our cortices are highly structured yet adaptive, allowing our neuronal activity to be dynamic and context specific. Much of this dynamic activity can be attributed to the projections of a small pontine nucleus: the noradrenergic locus coeruleus (LC).
Research into the LC and its role in Parkinson’s Disease is currently being driven by Dr. Claire O’Callaghan at the University of Sydney. In critical periods of learning and memory, LC neurons have shown to activate and project widespread across the brain.
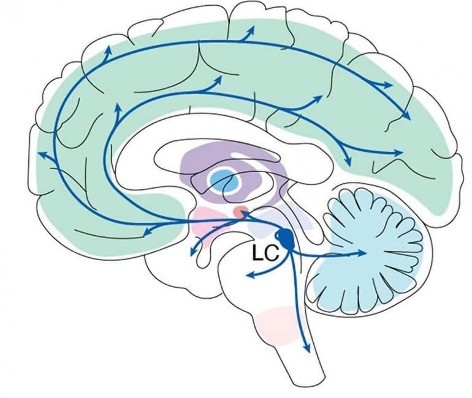
The locus coeruleus, situated in the dorsal pons, is a major source of noradrenalin and sends widespread projections throughout the brain
When considering primate evolution, the neuronal cell mass of the LC differs significantly. In comparison to humans who have ~ 50,000 LC neurons, chimpanzees (Pan troglodytes), considered one of our closest living relatives, have ~ 30,000 LC neurons. Interestingly, these number of neurons have been shown to be proportional to medulla, cerebellum and neocortical grey matter size.
Understanding phylogenetic variations is essential to understand how neural circuits and their targets have evolved. Many avenues remain to be explored but exploring similarities and differences together may allow us to begin unravelling the mystery of human behavioural evolution.
Written by Fernando Tinoco, a PhD student at the university of Sydney
