
Conjuring water from thin air — A beetle's guide to innovation
In the vivid and much-loved words of Dorothea Mackellar, Australia is "a sunburnt country, a land of sweeping plains, of rugged mountain ranges, of droughts and flooding rains”. Drought and water scarcity are an all too familiar struggle for Australians - especially for our farmers.1 This is not just an Australian struggle but a global one. Already, over 2 billion people live in countries experiencing high water stress,2 and approximately half of the global population live in regions susceptible to water scarcity.3 Climate Change is expected to make things worse.4
Clearly, water scarcity is a serious issue, but what if I told you that water could be conjured out of thin air? It sounds like magic, and one would probably say it sounds too good to be true, however, some species of Namib Desert Beetles do just that!
The beetles extract water from fog using a pattern of bumps and channels on their shells. The bumps are hydrophilic (water loving) and the channels are hydrophobic (water fearing), working in tandem to harvest water from the air. The hydrophilic bumps accumulate droplets of moisture from the air, then the beetle leans forward and the hydrophobic channels allow it to drip into its mouth.
Researchers have been studying how the shape and texture of the beetle’s shell helps to harvest water, and thus understand how we might make man-made surfaces that are able to do the same. These innovations rely on the interplay between hydrophilic and hydrophobic surfaces to collect and transfer water.
Hydrophobicity is the reluctance of water to spread out on or wet a surface. Hydrophobicity can be understood by thinking about how oil and water don’t mix - also known as immiscibility. Water is what is known as a ‘polar’ substance, and each molecule has a slightly negative charge on its oxygen atom and a slightly positive charge on its hydrogen atoms. This creates a strong attraction between water molecules through what we call ‘hydrogen bonds’. Substances must also be polar in order to mix with water. Oil molecules are ‘non-polar’, with no–or minimal–charge separation across the molecule. They are unable to participate in the water’s network of strong hydrogen bonds, and as a result do not mix with water - in fact the water molecules organise their hydrogen bonds to avoid interactions with the oil molecules, which also results in less interactions/mixing between the two.
A hydrophobic surface is non-polar like this oil – the water and the surface are unable to ‘mix’ and this leads to the water minimising its contact with the surface, creating a higher contact angle (or more rounded droplet) (figure 1).
Conversely, hydrophilicity refers to the tendency of water to spread out on or wet a surface. Hydrophilic surfaces are composed of polar molecules, allowing them to interact with polar water molecules through hydrogen bonding (if they have a suitable chemical structure) and/or through interactions between the charged parts of the molecules (called dipole-dipole bonding). These energetically favourable interactions between the molecules in the water and the molecules on the surface encourage the water to spread out or ‘wet’ the surface more in order to increase the number of these favourable interactions (figure 1).
This is a somewhat simplified explanation of hydrophobicity/hydrophilicity. If you are interested in finding out a more detailed explanation, I recommend reading The real reason why oil and water don’t mix.6
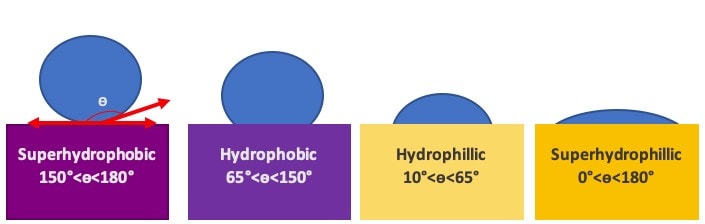
Figure 1: Diagram showing how water droplets interact with hydrophobic and hydrophilic surfaces including differences in contact angle, ɵ. Nano-scale roughening of a hydrophobic or hydrophilic surface can accentuate the surface’s hydrophilicity or hydrophobicity making it superhydrophilic or superhydrophobic.
Researchers have attempted to collect water from fog, and help tackle water scarcity, by developing various bioinspired structures that emulate the beetle’s shell. These structures are as simple as a hydrophobic copper mesh bound to hydrophilic cotton7, as well as sustainable cellulose films with hydrophilic and hydrophobic regions.8 These technologies also go beyond mitigating water scarcity and include the development of materials for separating oil-water emulsions, with potential industrial applications,9 and materials that control the adhesion of proteins, cells and bacteria to surfaces, which could be useful for tissue engineering or studying how cells communicate with each other. 10
The humble beetle shows just how much humanity can be inspired by, and learn from, nature to help solve daunting challenges, and innovate for the future.
Reference:
1 Institute for Sustainable Futures, The University of Technology Sydney. Water Scarcity Risk for Australian Farms & The Implications for the Financial Sector; Sydney, Australia: 2019
2 United Nations Water. Water Scarcity
3 International Institute for Applied System Analysis. Water Futures and Solution Fast Track Initiative Final Report; Laxenburg, Austria: 2016
5 Clegg, B. Oleic acid, Chemistry World.
6 Silverstein, T.P. The Real Reason Why Oil and Water Don’t Mix. J. Chem. Educ. 1998, 75(1), 116-118.
7 Cao, M; Xiao, J; Yu, C; Li, K; Jiang, L. Hydrophobic/Hydrophilic Cooperative Janus System for Enhancement of Fog Collection. Small. 2015, 11(34), 4379-4384
8 Xu, C; Feng, R; Song, F; Wang, X-L; Wang, Y-Z. Desert Beetle-Inspired superhydrophilic Superhydrophobic Patterned Cellulose Film with Efficient Water Collection and Antibacterial Performance. ACS Sustainable Chem. Eng. 2018, 6, 14679-14684.
9 Zeng, X; Qian, L; Yuan, X; Zhou, C; Li, Z; Cheng, J; Xu, S; Wang, S; Pi, P; Wen, X. Inspired by Stenocara Beetles: From Water Collection to High-Efficiency Water-in-Oil Emulsion Separation. ACS Nano. 2017. 11, 1, 760-769.
10 Ueda, E; Levkin, P.A. Emerging Applications of Superhydrophillic-Superhydrophobic Micropatterns. Adv.Mater. 2013, 25, 1234-1247.