
Advances in solar panel technology
There is an enormous fusion reactor in our planet’s sky. In just one hour, this reactor bathes the Earth’s surface in enough energy to supply all humanity’s electricity needs for a whole year. The problem is, the Sun’s energy arrives as solar radiation but we need to turn it into electricity.
The most direct way to make the conversion right now is with solar panels, but there are other reasons why they’re the great hope of renewable energy.

Here comes the sun. Professor Anita Ho-Baillie is working on the next generation of solar cells that will go beyond silicon and beyond current efficiency limits.
Their key component, silicon, is the second most abundant substance on Earth after oxygen. Since panels can be put where the power is needed – on homes, factories, commercial buildings, ships, road vehicles - there’s less need to transmit power across landscapes; and mass production means solar panels are now so cheap the economics of using them are becoming inarguable.
According to the International Energy Agency’s 2020 energy outlook report, solar panels in some locations are producing the cheapest commercial electricity in history.
Even that traditional bug-bear “what about when it’s dark or cloudy?” is becoming less problematic thanks to transformative advances in storage technology.
Moving beyond the limits of solar
If you’re expecting a but, here it is: but silicon solar panels are reaching the practical limits of their efficiency because of some quite inconvenient laws of physics. Commercial silicon solar cells are now only about 20 percent efficient (though up to 28 percent in lab environments. Their practical limit being 30 percent, meaning they can only ever convert about a third of the Sun's received energy into electricity).
This means that solar panel technology must soon evolve. A world leader in helping that evolution take place is Professor Anita Ho-Baillie who was recently appointed as the inaugural John Hooke Chair of Nanoscience, a philanthropically funded position by the late John Hooke CBE. Talking to her at the University’s Sydney Nano Institute labs, she points out another problem with using silicon.
“Solar panels need silicon that’s 99.9999% pure, but you start with an impure rock called quartzite. The purifying has to be done in four steps and each step involves heating to 1000 degrees Celsius. When I realised that I went ‘wow. That’s a lot of energy’.”
Still, a solar panel will produce many times more emissions-free energy in its lifetime than was used in its manufacture.
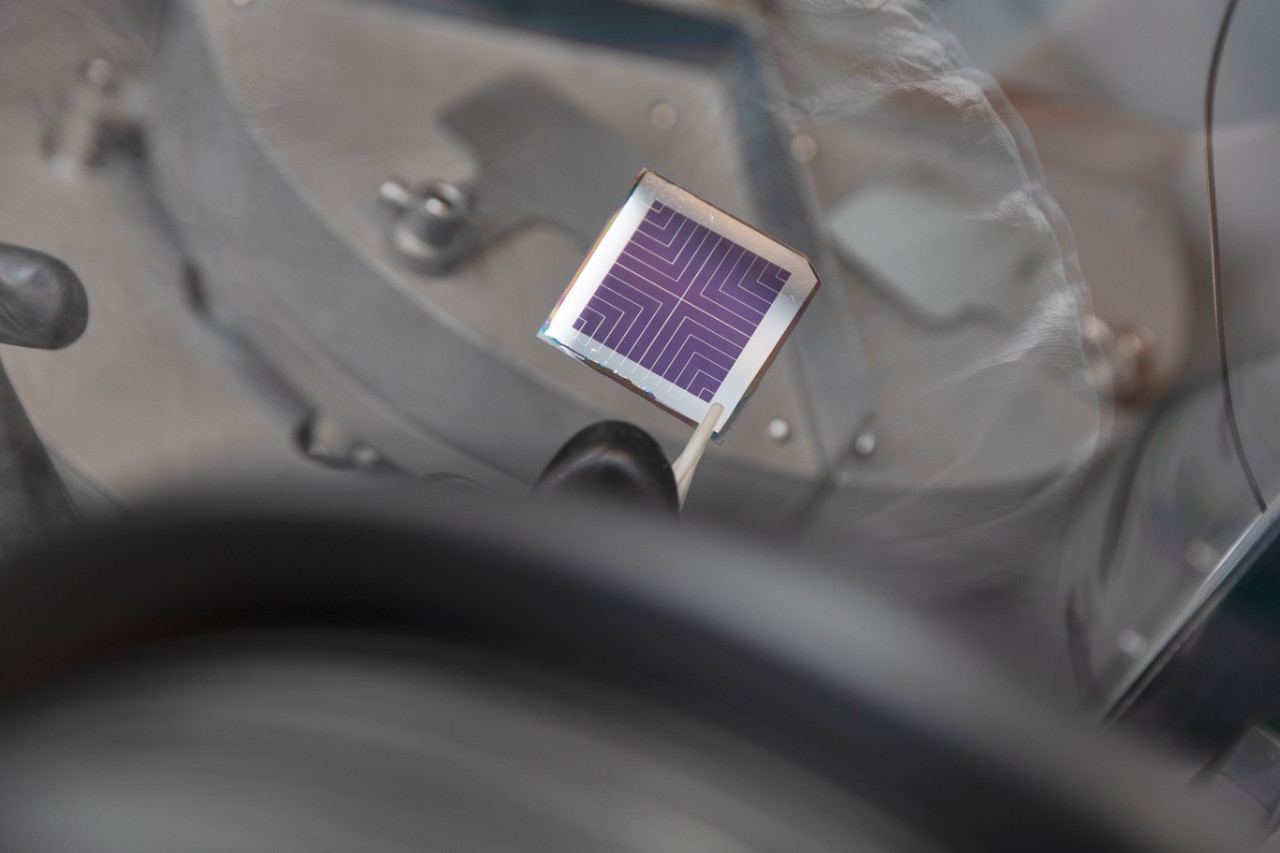
A perovskite solar cell. Peroskite cells are far easier and cheaper to produce than silicon cells, but they are much more easily degraded by moisture and heat.
You might not expect a world expert in materials engineering, semi-conductor physics, applied physics and chemistry to be playful and outgoing, but that’s how Ho-Baillie is. Hearing her talk about her career (including stints at British Aerospace, the telco Alcatel Australia and various solar-related organisations), you get the sense of someone who is quickly recognised by industry people as an asset worth having.
Underpinning all that is a hard-earned resourcefulness and independence linked to the handover of Hong Kong to China in 1997. As her parents made arrangements ahead of the handover to move to Sydney from Hong Kong, where Ho-Baillie was born, they sent teenaged Ho-Baillie ahead to continue her education.
“My parents didn’t go to university,” says Ho-Baillie. “But my mum said, ‘do a ‘PhD ‘cause you’ll be Dr Ho and I can tell my friends. And she does.’”
Set up in a flat on her own and knowing no-one, Ho-Baillie navigated her new country and excelled at her studies, despite her isolation. “I also cooked a lot of spag bol,” she says now, not really enjoying the memory.
It used to take me four weeks to make a silicon cell in the lab. With perovskite, it takes only two days.
As she developed her expertise, one of her early solar contributions concerned another little-known aspect of using solar panels; not all solar panels are compatible.
To get maximum output from a solar panel array, all the solar cells must be connected to other cells that match their natural characteristics, a laborious process. For her undergraduate thesis, Ho-Baillie created an algorithm that allowed mixed cells to be connected and still achieve maximum output.
“Imagine a factory that produces hundreds of cells a minute, and my goodness, that’s a lot of sorting they don’t have to do anymore,” Ho-Baillie says.
It's tricky: a silicon/perovskite solar cell
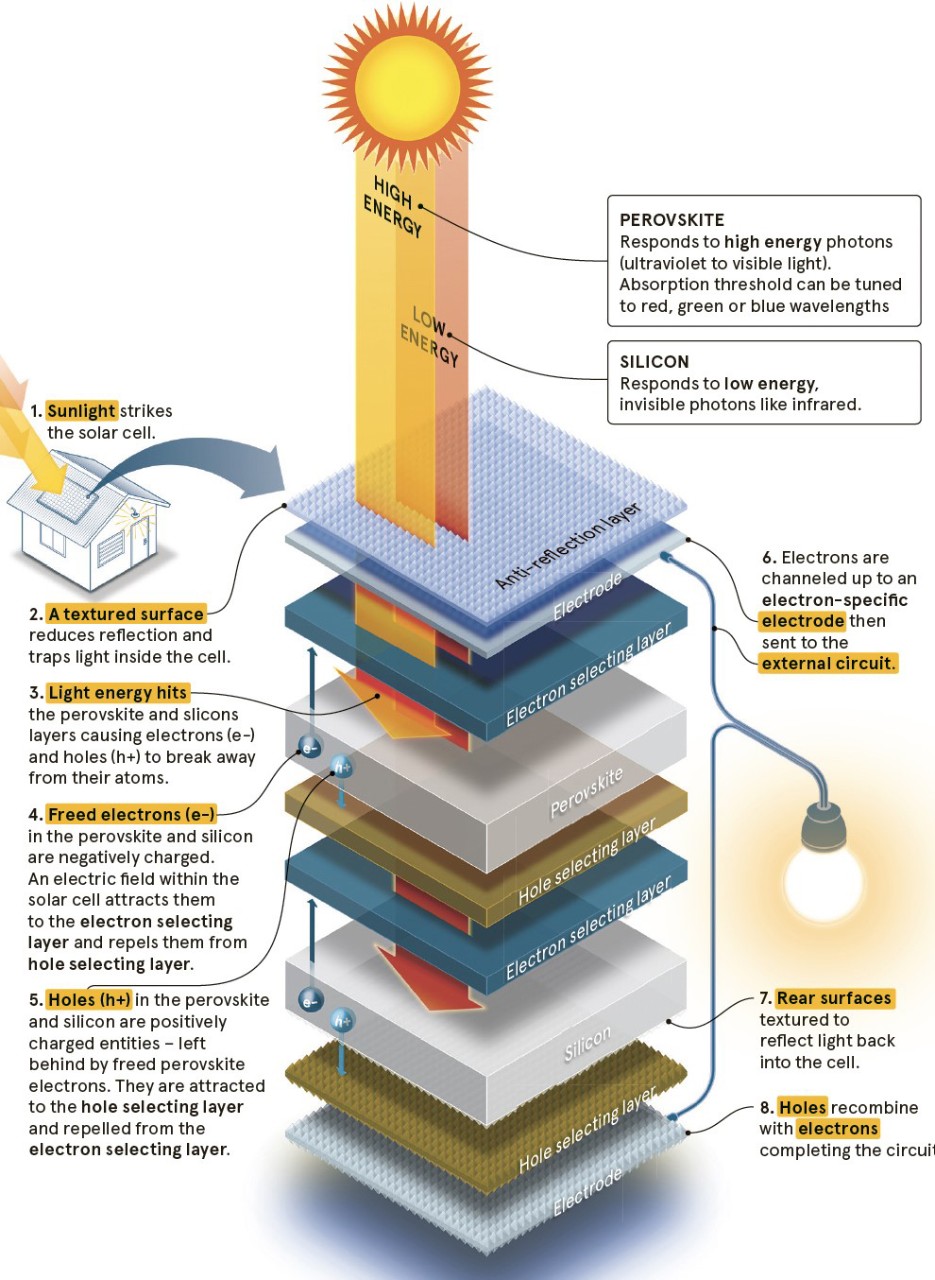
Electricity is made of electrons. Trouble is that most electrons are attached to atoms. All the various methods of electricity generation are about getting electrons away from their atoms. Solar cells do this using the energy in sunlight. Sunlight hitting silicon or perovskite knocks electrons loose which are channeled away to the external circuit.
Perovskite: the future of renewables
Now Ho-Baillie has turned her mind to creating the next renewable evolution. The substance that has become the focus of her research, and research around the world, is part of a class of crystalline compounds called perovskite; specifically, metal halide perovskite.
Like silicon, this crystalline substance is photoactive, meaning that when it’s hit by light, electrons in its structure become excited enough to break away from their atoms (this freeing of electrons is the basis of all electricity generation, from batteries to nuclear power plants). Given that electricity is in effect, a conga line of electrons, when the loose electrons from silicon or perovskite are channelled into a wire, electricity is the result.
An immediate benefit of perovskites for Ho-Baillie is that it saves time. “It’s just easier to handle than silicon,” she says. “It used to take me four weeks to make a silicon cell in the lab. With perovskite, it takes only two days.”
That’s because perovskite is a simple mixture of salt solutions that is heated to between 100 and 200 degrees to establish its photoactive properties.
Like ink, it can be printed onto surfaces, and it’s bendable in a way that rigid silicon isn’t. Being used at a thickness of up to 500 times less than silicon, it’s also super-light and can be semi-transparent. This means it can be applied to all sorts of surfaces like on phones and windows. The real excitement though, is around perovskite’s energy production potential.

Ho-Baillie in her lab. Ho-Baillie's team recently produced the first perovskite cell to ever pass the industry standard solar panel test set by the International Electrotechnical Commission. And it passed comfortably.
Overcoming perovskite's biggest challenge - deterioration
The first perovskite devices in 2009 converted just 3.8 percent of sunlight into electricity. By 2020, efficiency was 25.5 percent, close to silicon’s lab record of 27.6 percent. There is a sense that its efficiency could soon reach 30 percent. “It took people 40 years to double the efficiency of silicon,” say Ho-Baillie. “Perovskite caught up with silicon in just 10 years.”
If you’re expecting a ‘but’ about perovskite, well, there are a couple. A component of the perovskite crystalline lattice is lead. The quantity is tiny, but the potential toxicity of lead means it is a consideration. The real problem is that unprotected perovskite easily degrades through heat, moisture and humidity, unlike silicon panels which are routinely sold with 25-year guarantees.
“That’s the biggest challenge,” says Ho-Baillie. “You really want it to be long lasting if you’re going to put it on buildings or in solar farms.”
I love working with such bright young people [students]. They’ll be able to go out and change the world
It’s the work Ho-Baillie and her team are doing in this area that has recently captured world attention. The goal was for a perovskite cell to pass the industry-critical heat and humidity test set for solar panels by the International Electrotechnical Commission. The Ho-Baillie device was the first to pass, and it passed comfortably.
The innovation that made it possible was to laminate the perovskite cell with glass and the sort of polymers used in double-glazing windows. It was cheap and easy to do and, as it turned out, effective.
“This worked because as perovskite breaks down it starts to release gas – we actually call it outgassing,” says Ho-Baillie. “We found, if you laminate the cell onto a piece of glass with polymer so the cell is sealed off, the gas has nowhere to go. This prevents the outgassing, and so the breakdown, from happening at all.”
This has given a huge boost to the prospects of perovskite and seen Ho-Baillie become highly cited by researchers internationally. The timing is good, too, because the past few years have offered something that could produce the best solar cell efficiency ever seen. It’s called silicon perovskite tandeming where the two substances are layered into the same cell to give a higher voltage than either could give on its own.
This works because silicon is better at dealing with low-energy light waves, and perovskite works well with higher-energy visible light. Perovskite can also be tuned to absorb different wavelengths of light – red, green, blue. With careful aligning of silicon and perovskite, this means each cell will turn more of the light spectrum into energy.
The numbers are impressive: a single layer could be 33 percent efficient; stack two cells, it’s 45 percent; three layers would give 51 percent efficiency. These sorts of figures, if they can be realised commercially, would revolutionise renewable energy.
Asked about the most fun part of her job, Ho-Baillie doesn’t hesitate, “The students,” she says. “I love working with such bright young people. They’ll be able to go out and change the world.”
It’s the same with the work happening in Ho-Baillie’s lab at the University’s Nanoscience Hub. It’s entirely possible it will change the world.
Written by George Dodd for the Sydney Alumni Magazine. Photography by Louise M Cooper.