
Hydrogen embrittlement in steel and a cure in atom probe tomography
Add carbon to iron, you get steel. Add hydrogen to steel, you get a potential catastrophe. The condition is hydrogen embrittlement and it has so far resisted all attempts at an answer. Professor Julie Cairney is looking closely at the problem.
You might never have heard of hydrogen embrittlement (HE), but you have definitely heard of its consequences.
It was implicated in the 2011 Fukushima Daiichi nuclear disaster, the Deepwater Horizon oil spill in the Gulf of Mexico (the largest marine oil spill in history) and countless other structural failures affecting aviation, shipping, construction and energy production.
It affects a range of metals, but it’s a big problem with steel, like the steel in the screws that were holding up your cupboard that suddenly dropped off the wall for seemingly no reason.
Since it was first identified in 1875, HE has been a focus of international research, but no solution has emerged.
As the name hydrogen embrittlement suggests, the villain here is hydrogen. If it diffuses its way into steel during the manufacturing process or through corrosion, the steel becomes brittle. Strangely, the stronger the steel, the more likely it is to eventually suffer from HE. The question is, what does hydrogen do to the atomic structures of the susceptible metals?
The answer could come through an exquisitely precise technology called atom probe tomography and the work of Julie Cairney who is a professor in the School of Aerospace, Mechanical and Mechatronic Engineering at the University and Director of the Australian Centre for Microscopy and Microanalysis.
In effect, Cairney and her team of PhD students and early career researchers are explorers of the impossibly small worlds that have been opened up by advances in microscope technology.
Professor Julie Cairney studies materials using advanced microscopy techniques that can image matter down to atomic scale.
“I first used a microscope in my final undergraduate year of Materials Engineering, for a subject called failure analysis,” says Cairney, who has an easy energy and a talent for communicating ideas. “We looked at some actual bone replacement implants that had failed inside someone’s body.
“Through a scanning electron microscope, they looked beautiful and said so much about why the failure happened. We then wrote a diagnosis of the failure for the technicians at the hospital.”
Techniques in atom probe tomography
The word ‘microscopic’ slightly underplays the places where Cairney does her work. She observes the behaviour of actual atoms, with atom probe tomography (APT) allowing the creation of 3D images of clusters of atoms and how they connect with each other, including where hydrogen atoms might rest destructively in the atomic structure of steel.
Describing atom probe tomography takes us into an area of deep science. To demonstrate that it is used with any number of substances, including steel, the following explanation is based on a substance previously studied by Cairney’s team: tooth enamel.
The first step is to form the tooth enamel into a super-fine needle. Atoms like to move around so the needle is put into an ultra-high vacuum chamber and the temperature reduced to a stabilising 100 kelvin (which is more than -170 degrees Celsius).
The needle is pointed at an ion detector, which is not unlike a home smoke detector responding to ion-rich smoke. Applying an electrical pulse to the needle unbinds one atom, making it an ion. When the first ion hits the ion detector, progressively followed by others, the puzzle starts piecing together.
Different ions can be light or heavy, so the time an ion takes to reach the detector identifies what element it is. The detector also records where the ion hits, allowing its position in the needle to be calculated. This information creates a 3D image showing how the different substances in the tooth enamel are arranged atomically.
Looking at the 3D image, Cairney’s team saw something unexpected. They saw magnesium.
It was already known that tooth enamel is made of rods of a crystalline substance called hydroxyapatite. What Cairney’s team discovered was magnesium discretely trimming the rods. The rods themselves are only 20 nanometres long (for scale, a piece of paper is 75,000 nanometres thick), so maybe it’s not so surprising that such a tiny amount of magnesium was missed.
With this insight, researchers can now think differently about how tooth enamel is formed, the pathways that cause decay and how to regenerate it. This list is not unlike the questions around the current steel project which has a particular relevance for Cairney, who has a background in the science of metallurgy.
Professor Cairney offers her expertise in the three-dimensional mapping of atoms through the University's core research facility: Sydney Microscopy & Microanalysis.
Metals under the microscope
Cairney was born and raised in the mining city of Broken Hill in far western New South Wales, “The professional people you meet in mining towns are likely to be geologists or metallurgists or mining workers,” she says. “That was the industry that I grew up with and all that geology stuff is so fascinating to me.”
What made it possible for Cairney to study the subject she loves was a scholarship. She remains a strong advocate for helping young people through their study, “Scholarships are a powerful way of fighting disadvantage,” she says.
Now a leader in her discipline, Cairney is ideally placed to investigate the mystery of hydrogen embrittlement. Part of the reason an answer is so elusive is because hydrogen is the smallest of all atoms and won’t stay put long enough to be observed, even by APT. By the time the needle of steel has been prepared, the hydrogen is gone. But Cairney and her team might have found a solution.
“There is another type of hydrogen called deuterium. It’s like standard hydrogen but it‘s heavier, so we can identify it, and it marks where the hydrogen is in the micro-features of the steel,” she says.
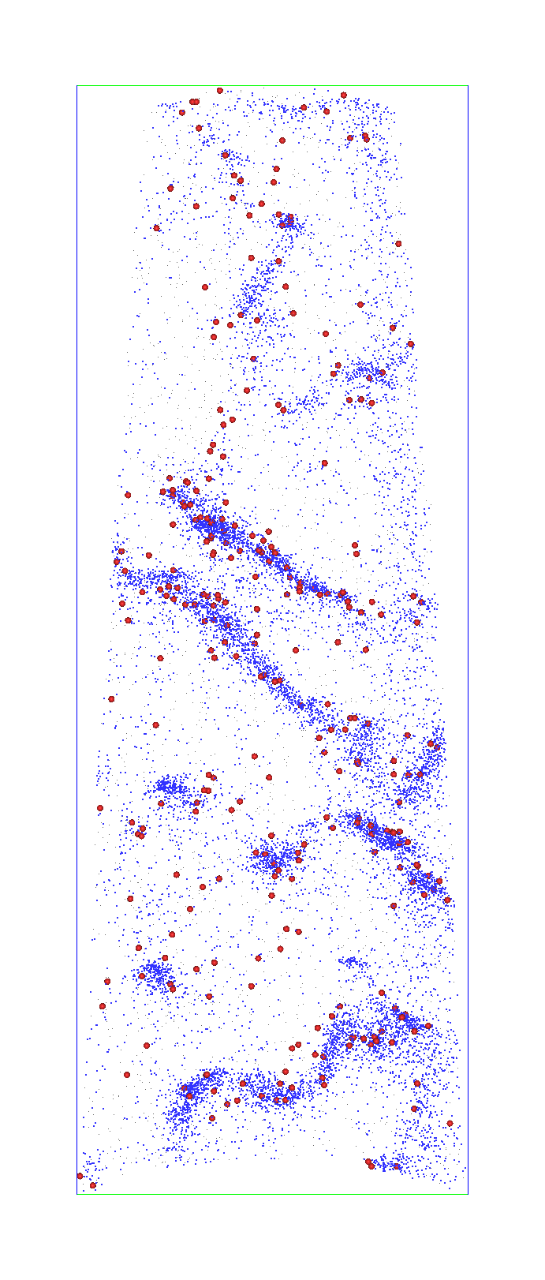
Observation of hydrogen atoms (red) in high-strength martensitic steel for automotive applications. The distribution of hydrogen atoms coincides with the carbon atom concentrations in steel's defects.
Early results suggest problems may arise when hydrogen atoms settle in load-bearing locations or where metal needs to be bendable. Every piece of new knowledge brings a solution closer. While the key goal is improving safety and reliability in construction, there could be other benefits.
“If you can use higher strength steel to make cars because you know it won’t become brittle, you can use less of it,” says Cairney. “That means lighter vehicles using less fuel and a massive reduction in emissions over time.”
Beyond the steel problem, Cairney’s team is also working towards what would be a dream project – working on samples brought back to Earth from a Mars mission. Currently, they’re in what could be termed an audition phase.
Working with Glasgow University, they’ve analysed Martian meteorites to see if they hold evidence of water on Mars, which they do. Now they’re confirming those results.
“We need to demonstrate that our team can provide accurate information so that we can get to work on actual samples from Mars,” says Cairney with obvious excitement. “That’s the thing that I absolutely love the most about my job, being able to be part of the process of discovery.”
Banner image: Illustration by Nigel Buchanan.